(CDSCO) approves India’s first Chimeric Antigen Receptor (CAR)-T cell therapy to treat blood cancer
Therapy, called NexCAR19 (Actalycabtagene autoleucel) is the first humanized CD19-targeted CAR-T cell therapy.
This therapy is intended for patients with CD19 biomarker in B cells, a protein used to diagnose cancers stemming from B cells, such as acute and chronic lymphoblastic leukaemia (blood cancer).
Who is developed CAR-T Cell Therapy?
It is developed by Immunoadoptive Cell Therapy (ImmunoACT), an IIT Bombay incubated company.This CAR-T cell therapy will put India on global advanced cell-and -gene therapies map.
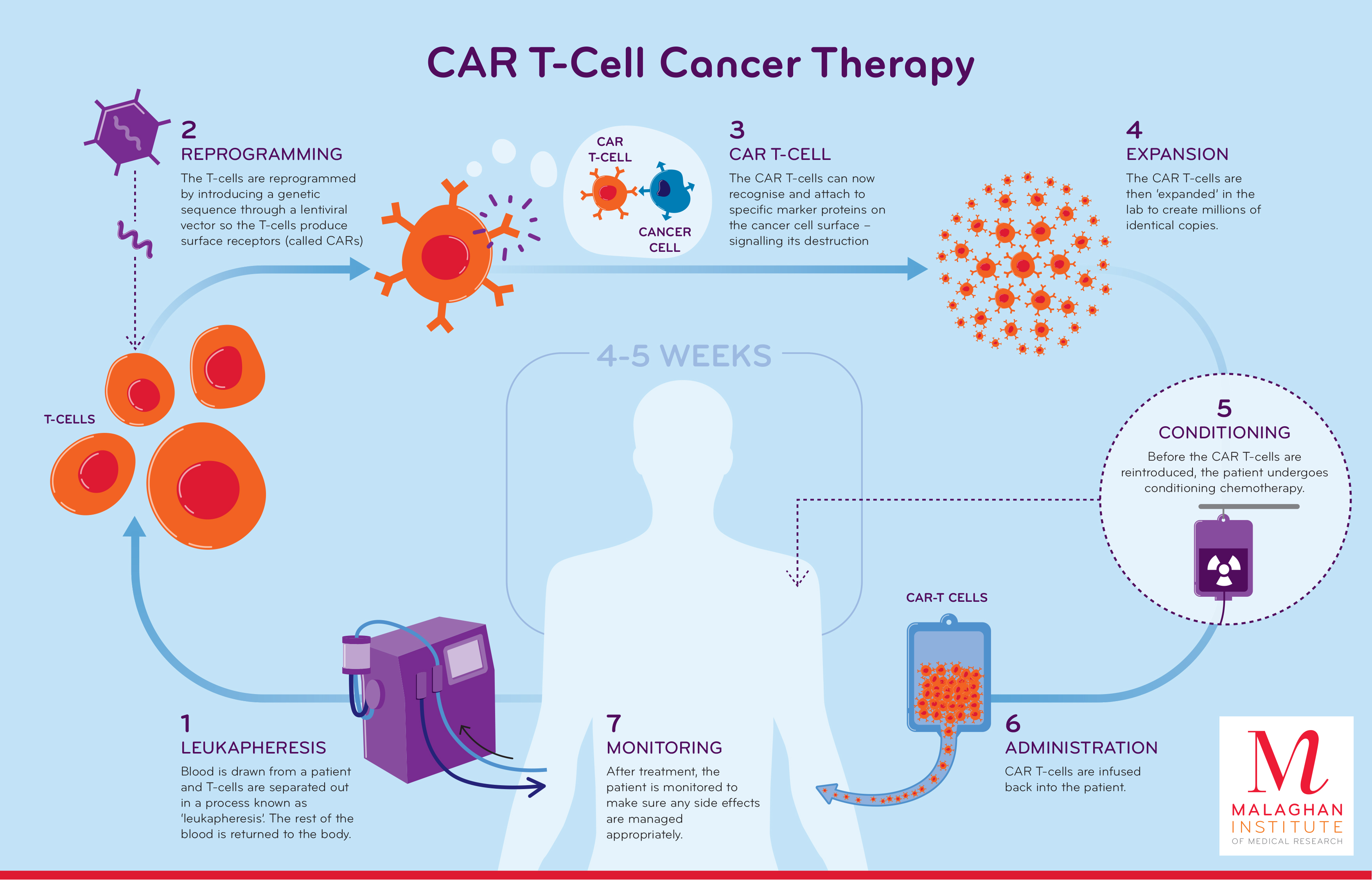
How does CAR-T Cell Therapy Work?
- CAR T cell therapy is a type of cellular immunotherapy treatment that uses T cells that are genetically altered in a lab to enable them in locating in destroying cancer cells more effectively.
- T cells are immune cells that attack infectioncausing pathogens (viruses, bacteria, fungi and parasites) and harmful cells, like cancer cells.
Significance of CAR-T Cell Therapy
- Short treatment time needed because aggressive chemotherapy is not used.
- Prolonged durability, since T cells can persist in body for long-term, they may recognize and attack cancer cells.
About CDSCO
CDSCO (under Ministry of Health and Family Welfare) is the Central Drug Authority for discharging functions
assigned to Central Government under Drugs and Cosmetics Act, 1940.
Major functions: Regulatory control over import of drugs, approval of new drugs and clinical trials etc.
